|
|
10/12/2010 |
 In the past couple of years, we have done lots and lots of testing and research to figure out why in some cases dewaxed shellac flakes won't dissolve in alcohol. The problem with our testing is so far we haven't found any shellac we can't dissolve in a reasonable amount of time. In the picture above are two bottles of freshly dissolved shellac. We started out with the oldest chunk of shellac flakes we could find, a gnarly block that was too clumpy to sell to customers and was lying open in a hot, humid warehouse for at least two years - more if you include the time before it came here. We broke off chunks and put them in the bottle on the left. In the bottle on the right, we took the same shellac, but pulverized the chunks first into a coarse powder (see picture below). For the first couple of hours we aggressively agitated both samples every half hour or so. In the jar with the pulverized stuff the bits eventually gelled together on the bottom of the jar. We broke up the gelled bit with a handy screwdriver, agitated it some more and went on with our lives. We went home. The next morning, both bottles were dissolved except for a tiny bit on the bottom (in both samples) and a little more agitation took care of that. We used the shellac with no issue for French polishing. In the past couple of years, we have done lots and lots of testing and research to figure out why in some cases dewaxed shellac flakes won't dissolve in alcohol. The problem with our testing is so far we haven't found any shellac we can't dissolve in a reasonable amount of time. In the picture above are two bottles of freshly dissolved shellac. We started out with the oldest chunk of shellac flakes we could find, a gnarly block that was too clumpy to sell to customers and was lying open in a hot, humid warehouse for at least two years - more if you include the time before it came here. We broke off chunks and put them in the bottle on the left. In the bottle on the right, we took the same shellac, but pulverized the chunks first into a coarse powder (see picture below). For the first couple of hours we aggressively agitated both samples every half hour or so. In the jar with the pulverized stuff the bits eventually gelled together on the bottom of the jar. We broke up the gelled bit with a handy screwdriver, agitated it some more and went on with our lives. We went home. The next morning, both bottles were dissolved except for a tiny bit on the bottom (in both samples) and a little more agitation took care of that. We used the shellac with no issue for French polishing.
We were therefore very puzzled about the problems Vijay Veiji, the author of an article about shellac in the December 2010 issue of Fine Woodworking, had dissolving his shellac. We were also puzzled about his claim that dewaxed shellac expires after a couple of years and if it dissolves it will take longer to dry. His problem is pretty common but easily solved. As most people have seen, if you spill some scotch on an old French polished table, you will dissolve and damage the finish pretty easily. So age really isn't the main issue. The issue he clearly demonstrates in the article is the importance of agitation. When you have small flakes of shellac and don't agitate, you get the following problem: the flakes start to dissolve, glom together, form a gel and the alcohol cannot penetrate the gel. However agitation and breaking up the gel will solve this problem. Anhydrous alcohol speeds it along. The problem is exacerbated by the hardware store alcohol Mr. Veiji uses - all those molecules of water in the alcohol block the alcohol from contacting the shellac, and result - even after dissolving - in a softer finish. If he used 200 proof anhydrous ethanol he would not have the problem of slower dry times and a softer finish simply because hardware store alcohol has water which gets trapped and leaves a softer finish and other alcohols which have a higher flash point and simply evaporate slower.
 Another thing to mention is that oxidation happens on the surface of the shellac. Bigger flakes, like the ones you get in our Tiger Flakes, have less surface area than lots of small flakes. Also, bigger flakes have more gaps for alcohol. Breaking up shellac exposes fresh surfaces, which is good. When we dissolved our older flakes, we thought that if there really were any substantial amount of shellac that could not dissolve, we would get residue in the container. But we didn't because the actual layer of oxidation is not more than a molecule or three and just disappears. Another thing to mention is that oxidation happens on the surface of the shellac. Bigger flakes, like the ones you get in our Tiger Flakes, have less surface area than lots of small flakes. Also, bigger flakes have more gaps for alcohol. Breaking up shellac exposes fresh surfaces, which is good. When we dissolved our older flakes, we thought that if there really were any substantial amount of shellac that could not dissolve, we would get residue in the container. But we didn't because the actual layer of oxidation is not more than a molecule or three and just disappears.
Now, I will not argue that oxidation of the shellac flakes doesn't take place - just that it happens, is normal, and isn't a big deal. You can avoid the issue by using proper agitation; anhydrous alcohol; larger shellac flakes; and storage in a non-permeable container in a cool and dry place. We are now packing our shellac with desiccants that help with the water vapor and pack in a resealable, nylon-impregnated bag which is less permeable than before. By the way, most of the professionals we know who use flake shellac on a regular basis also use mechanical agitation to help lower dissolve times. It's not a requirement but it makes it easier.
In addition to buying your shellac directly from us, you can also buy Tiger flakes from Woodcraft.
Note: In our continuing quest for knowledge and experience, we want to know if you do have any flakes that you cannot dissolve. Please send them to us and let us try to dissolve them. I would love to find a sample that won't dissolve so I have a control for our other testing.
Epilogue: (April 2020) In the past 10 years we have found some shellac that we cannot dissolve. Usually it is many years old, and more refined varieties (Blond and Super Blond) have a shorter shelf life than Garnet. Another factor in the shelf life of the flakes is how it is stored. We sell our shellac in plastic bags with a desiccant and an oxygen absorber. The plastic bag, while a decent barrier isn't totally impermeable and shellac left in the bags for years will deteriorate and eventually not dissolve. The packaging is designed for long shelf life in a store, not long term storage in a workshop. Braking up the flakes can help. Shellac should be stored in an impermeable container (glass) and stored in a refrigerator or other cool environment. |
Join the conversation |
|
 Joel's Blog
Joel's Blog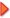 Built-It Blog
Built-It Blog Video Roundup
Video Roundup Classes & Events
Classes & Events Work Magazine
Work Magazine


 In the past couple of years, we have done lots and lots of testing and research to figure out why in some cases dewaxed shellac flakes won't dissolve in alcohol. The problem with our testing is so far we haven't found any shellac we can't dissolve in a reasonable amount of time. In the picture above are two bottles of freshly dissolved shellac. We started out with the oldest chunk of shellac flakes we could find, a gnarly block that was too clumpy to sell to customers and was lying open in a hot, humid warehouse for at least two years - more if you include the time before it came here. We broke off chunks and put them in the bottle on the left. In the bottle on the right, we took the same shellac, but pulverized the chunks first into a coarse powder (see picture below). For the first couple of hours we aggressively agitated both samples every half hour or so. In the jar with the pulverized stuff the bits eventually gelled together on the bottom of the jar. We broke up the gelled bit with a handy screwdriver, agitated it some more and went on with our lives. We went home. The next morning, both bottles were dissolved except for a tiny bit on the bottom (in both samples) and a little more agitation took care of that. We used the shellac with no issue for French polishing.
In the past couple of years, we have done lots and lots of testing and research to figure out why in some cases dewaxed shellac flakes won't dissolve in alcohol. The problem with our testing is so far we haven't found any shellac we can't dissolve in a reasonable amount of time. In the picture above are two bottles of freshly dissolved shellac. We started out with the oldest chunk of shellac flakes we could find, a gnarly block that was too clumpy to sell to customers and was lying open in a hot, humid warehouse for at least two years - more if you include the time before it came here. We broke off chunks and put them in the bottle on the left. In the bottle on the right, we took the same shellac, but pulverized the chunks first into a coarse powder (see picture below). For the first couple of hours we aggressively agitated both samples every half hour or so. In the jar with the pulverized stuff the bits eventually gelled together on the bottom of the jar. We broke up the gelled bit with a handy screwdriver, agitated it some more and went on with our lives. We went home. The next morning, both bottles were dissolved except for a tiny bit on the bottom (in both samples) and a little more agitation took care of that. We used the shellac with no issue for French polishing.  Another thing to mention is that oxidation happens on the surface of the shellac. Bigger flakes, like the ones you get in our Tiger Flakes, have less surface area than lots of small flakes. Also, bigger flakes have more gaps for alcohol. Breaking up shellac exposes fresh surfaces, which is good. When we dissolved our older flakes, we thought that if there really were any substantial amount of shellac that could not dissolve, we would get residue in the container. But we didn't because the actual layer of oxidation is not more than a molecule or three and just disappears.
Another thing to mention is that oxidation happens on the surface of the shellac. Bigger flakes, like the ones you get in our Tiger Flakes, have less surface area than lots of small flakes. Also, bigger flakes have more gaps for alcohol. Breaking up shellac exposes fresh surfaces, which is good. When we dissolved our older flakes, we thought that if there really were any substantial amount of shellac that could not dissolve, we would get residue in the container. But we didn't because the actual layer of oxidation is not more than a molecule or three and just disappears.
I use denatured ethyl alcohol and have had no problems with flakes not dissolving.
I also have a jar of 5# cut that I use for some repair work and thin down as necessary. This only took a short time more than my regular 2# cut to dissolve.
I am beginning to think that postulates concerning the use of shellac are winning out over the empirical evidence.
F.
Chuck - A rock tumbler would probably speed up dissolution of shellac, but I don't think it's really needed. If you needed your shellac 10 minutes ago, it might have a use, but I imagine there would be some theoretical danger from the motor sparking with (possibly non-extant) alcohol vapors around.
I haven't yet been able to try the anhydrous alcohol, but I plan to get some when I can.
Tor
Really nice to have you around.
Cheers
David
I, too, would find it interesting if you ever did find some that wouldn't dissolve. My money on each non-dissolving sample that gets returned is that the problem is user-related and not product-related.
The issues in dissolving shellac are common to dissolving polymers in a solvent so there is a vast amount of experience in this operation. The problem is that a gel layer forms at the surface and large molecules such as shellac (or polymers) are very slow to diffuse through this gel layer and into true solution. Dissolution will be hastened by mechanically breaking up this gel layer as it forms. Alternatively, one can diffuse solvent, which will diffuse rapidly, into the gel layer to gel the whole mass and then mechanically break up the easily dispersed gel.
If time is not important slow tumbling will get the job done. A commonly used approach is to put the polymer in a jar with solvent and then slowly roll the jar over night on a set of rollers. Putting a padded jar in the rock tumbler would do nicely if it turned at a safe speed. If time is important, then high speed mixing will relatively quickly dissolve shellac (or polymers) but high speed mixers are not a common shop accessory. Ideal is a magnetic mixer, a common lab appliance. If you can snag one of these on Ebay or elsewhere they are ideal for the task. Put shellac, solvent and magnetic stirring bar in a jar, sit jar on the mixer and in a couple of hours even large flakes are dissolved.
Small molecule solvents are never "trapped" in a thin polymer or shellac film. These small molecules can move rapidly about the film and they will diffuse out over time, more or less quickly, depending on film thickness, temperature and other factors. Perhaps the water in typical 95% ethanol has some effect on shellac but any water not chemically bound will leave the shellac film, and fairly quickly.
thank you for your post. I met Don just recently at WIA 2010 and I have much to learn from him.
Trapped water vapor (vapor not molecules) will eventually evaporate, but much slower than alcohol. So unless you apply shellac very thinly, or use anhydrous alcohol, the water vapor will leave pores in the shellac layer that result in a more permeable surface and a softer finish. In my next segment on shellac - this week - I go into this more.
A few observations from a professional finisher. I've made and applied hundreds of gallons of shellac over 25 years of finishing.Not once have I used anhydrous alcohol.
#1 Blonde shellac flakes have a shelf life. I have older flakes that would not dissolve properly through agitation or heat extraction. The processing that blonde shellac goes through shortens it's life span. It's simple science.
#2 Using anhydrous alcohol is not required unless you just want to spend more money. The amount of vapor trapped in a thin application of shellac would be hard to determine unless you do laboratory testing.The same goes for the hardness quotient.Besides the argument can be made that once opened, anhydrous alcohol is no longer anhydrous, unless you have absolute control over the humidity in your shop.
Finishing with shellac still has it's fallacies which keep getting passed around as doctrine.
In reference to the author of that article, Mr. Vieji; maybe you should contact him and have a professional discussion. It couldn't hurt.
#1 - You may have trouble dissolving blond shellac but look at the comments by myself and other posters in this and succeeding blog entries on shellac. We and they don't have this problem. In tests we could not find any sample shellac we could not dissolve in a reasonable time. If you think you have some please send it to me and let us try to dissolve it.
Better alcohol certainly makes the dissolve process easier.
In whatever historic literature I can find the recommendation for dissolving shellac is always 180 (or better) proof ethanol, the rest being water. So my guess is that it's the isopropal and methanol adulterants that give you the dissolving problem. (It's an easy test which I should do but haven't yet).
2 - if you open and close a bottle of anhydrous alcohol as you use it it will stay reasonably anhydrous. The best part of anyhydrous alcohol is that it makes things like a french polish more forgiving. Yes you can French Polish with regular ethanol - but it's easier with better stuff.
3 - Why anyone would voluntarily use any product with amounts of methanol in it is beyond me. In a separate workshop, with proper safety equipment your exposure can be kept to a minimum but in a basement shop your entire family will be exposed. And considering that finishing isn't an instant process for most amateurs it means there will be toxic fumes in the air for awhile. With French polishing of course, unless you always wear gloves there is skin absorption and I spoke with a turner today who was really happy about our alcohol because when you french polish on a spinning lathe you cannot wear gloves.
WE have already gotten comments from very very experienced French Polishers who have given our alcohol raves and say it is like the stuff you can get in France.
Interesting discourse. The alcohol I use is a high quality ethanol.I'm not sure what the % of adulterant it has but will find out and let you know.
Yes the shellac was old and did not dissolve well. If you'd care to send me some of your alcohol I will conduct an impartial test and report back to you. What do you consider a reasonable time for dissolving?
I noticed in your tests you french polished with shellac from that clump. What was it you FP? A small sample board does not pass the litmus test. I also wonder what the hardness and longevity of the film would be.
Reasonably anhydrous? That's interesting.Laboratories that use anhydrous alcohol require very controlled standards to keep it that way.Yet we can assume reasonably anhydrous is the same thing?
I know the dangers of methanol. It's also beyond me why people smoke, text while they drive etc etc.
You did address my comments except one major point. When you take a man to task isn't it reasonable to take it up with him personally? I'll bet you could contact Mr. Vieji as he has a website also. Why not do so? I would be very interested to hear the results of a one on one discussion.
I may be wrong but it seems to me someone who actually travels to India to buy shellac presumably has good contacts and knowledge in the industry. Might they be better informed than those who don't?
Do not take this question as impertinent but are you a professional finisher? I know you have people writing in but I'm asking what your field experience is. As a professional, I have French polished countless pieces of furniture and a number of rooms too. Talk about having your shoulders get weary!! I look forward to your response
usually with agitation we make good progress during the afternoon and let it sit overnight. A few shakes in the AM and all should be well. Most professional finishers I know use mechanical agitation of some sort so it goes faster.
> I noticed in your tests you french polished with shellac from that clump. What was it you FP? A small sample board does not pass the litmus test.
The point was to prove that the shellac was fine. Which it is. Shellac from the same batch was used to French Polish a desk. Which also came out fine. The sample in question was the very first bit of French Polishing the person who did it ever did. And it required very little, bordering on no, skill. The right materials make the job considerably easier.
> I also wonder what the hardness and longevity of the film would be.
We have not stressed the finish yet, but it is rock hard, really rock hard, and my guess, from other tests, reasonably water proof. With pure ethanol there are just fewer pores in the shellac.
-Reasonably anhydrous? That's interesting.Laboratories that use anhydrous alcohol require very controlled standards to keep it that way.Yet we can assume reasonably anhydrous is the same thing?
We try to work under actual conditions. once a bottle of alcohol is open is will absorb moisture from the air. keeping the jar normally closed minimizes this. Since I haven't measured the water percentage I can only suggest that it is a very small amount, but more than the original sealed bottle.
> I may be wrong but it seems to me someone who actually travels to India to buy shellac presumably has good contacts and knowledge in the industry. Might they be better informed than those who don't?
Buying shellac is completely different than using shellac. How shellac is made is interesting (although he left out the refining steps that are done in Germany) but really doesn't tell you much about using it. I found Mr. Vieji's article very interesting (although nothing I didn't know) and only took issue with his last paragraphs on shellac aging and mixing.
>
> Do not take this question as impertinent but are you a professional finisher?
No, but we have gotten affirming comments from more than one professional finisher (just not you :) )
> I know you have people writing in but I'm asking what your field experience is.
I am by training a mechanical design engineer. What the history of engineering is, and what engineers do, is try to turn a craft into a repeatable science. In this case by actually testing shellac and alcohol under a myriad of conditions we have far better understanding of how it ages, dissolves, and should be stored, than a working finishing professional whose main job it is is to finish things and doesn't have the time or the resources to to the experimentation we have to do. And that's what we do, it allows us to create better products, let people work more efficiently, safer, and easier.
Here's a test for the finish sample you made up. Tape off an area on it and pour some alcohol within that confinement. Let it sit for a minimum of ten minutes and then wipe it off. Please share your results.
I know it's splitting hairs when I talk about the % of water in anhydrous. I wonder under normal working conditions how long it stays as such. I have very controlled conditions in my shop but don't have the facility to do such testing.
I know the processing shellac goes through.It's what shortens it's life span. On that I guess we agree to disagree, but for me it's simple science.
My point of Mr. Vieji is that beyond buying and selling, as you do, he may a have deeper knowledge of the subject.
I would really like to hear a discourse between you and he. It would be interesting and enlightening.
Would you do it? Something tells me no but I'll bet it would be great for your blog.
Concerning a working professional who doesn't have time for testing: that was extremely presumptuous and haughty at the same time. It presumes I just happily slop finishes about without a "far better understanding" of it's properties. You have absolutely no idea how far off base you are with that statement.
I always found engineers the hardest to teach because of their rigidity.
I hope you post results on the alcohol test and the possible contact with Mr. Vieji. Back to my mindless finishing.
Actually it's a great test. Ethanol, Vs. Vodka Vs. store bought Alcohol. On a fairly fresh French Polished piece, and a piece a couple of years old.
I am sorry if you are offended when I say you don't have the time for testing. I don't think you slop finishes around at all. I am pretty sure you are an expert finisher. But how many different formulations of alcohol have you tested? How many different aging shellac tests have you run? How many shellac preservation tests have you done? It's not your job? It's ours, that's what we do for a living. There is a reason why all modern finishes are usually developed by chemists and engineers not working finishers.
ps - Please include a valid email in any future posts as otherwise I will not post them. I think at this point we are going around in circles anyway. When I do the test you suggest I will post the results.
PS: It is not my place, nor anyone else, to tell Joel how to run his blog or how to operate it, or complain. For those who don't like what he writes, kindly go away in peace. It is a concept known as, "hospitality." Homer's Odyssey can provide a more in depth understanding.
"The lightest color available by the solvent extraction process of removing the wax and color. This gentler process (as opposed to chemical bleaching) doesn't affect the durability."
I notice your lightest shellac is bleached. Should I be concerned?
Great blog, by the way, and I really appreciate the way you go about things.
Brian
As far as I know Jeff and I sell the same stuff - refined in Germany. We call it by different names. My guess is he is right - I'm wrong - and our stuff isn't bleached either - But I don't know for sure. When I wrote the description I didn't actually check with the manufacturer on the refining process - just copied what is commonly accepted for the shellac. So I am probably wrong and I need to do some checking.